At PRETOX CONSULTANCY (PTC), we provide comprehensive support in implementing and maintaining OECD Principles of Good Laboratory Practice (GLP) to ensure the highest level of data integrity, reliability, and regulatory acceptance for preclinical/nonclinical studies.
We are very well experienced with GLP system which is very important and critical aspect for non clinical studies.
PTC (Pretox consultancy) is highly understanding the impact of OECD Principles of Good Laboratory Practice (GLP) which form a rigorous quality system designed to ensure the integrity, reliability, and reproducibility of non-clinical safety studies—especially those used for regulatory submissions involving chemicals, pharmaceuticals, and other substances. GLP is a set of principles developed by the Organization for Economic Co-operation and Development (OECD) that governs how laboratories plan, perform, monitor, record, and report studies.
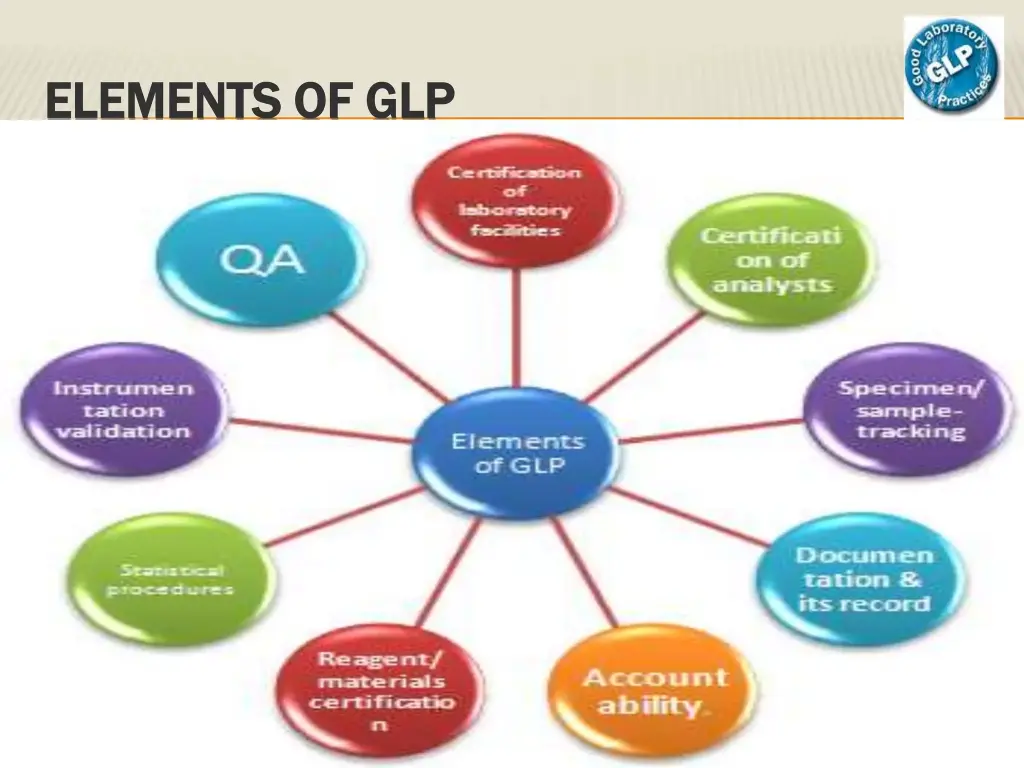
We have exposure about key elements of the GLP Quality System which definitely helping to monitor the preclinical studies:
- Test Facility Management
- Quality Assurance Program
- Study Director
- Standard Operating Procedures (SOPs)
- Study Protocols and study Report
- Principal investigator
- Data Recording & Reporting
- Archiving

